Between your business and the energy industry, there is room for energy storage.
In the near future, the investor who stores energy and sells or uses it when other suppliers are not producing it will win.
This is due to the fact that storage facilities can provide energy in a way that is better matched to the nature of the consumers, as well as serving the area-based balancing of energy flows.
Industries using energy storage

Energy storage facilities
in companies
In recent years, the operations of almost all companies, including industrial plants, have been undergoing significant changes in terms of how they meet their energy needs - the result of management aiming to improve the economic efficiency and ecology of their operations through modern technology, but also often a necessity caused by changes in the electricity market and in national and international law.
Why are energy storage facilities becoming an essential component of industrial plants?
Nowadays, global competition requires industrial plants to reduce their production costs and carbon footprint in every way possible and to protect themselves against losses in the event of power loss so that they can stay in business regardless of the circumstances. Tenders increasingly require compliance with EU green procurement criteria for electricity. In each of these cases, the use of energy storage can be a solution.
Economic benefits:
- Possibility to reduce the amount of energy drawn from the grid when working with RES and/or CHP installations;
- Protection against having to stop production in the event of a blackout;
- Smoothing of the energy demand profile, which makes it possible to reduce the power demanded - Peak shaving;
- Buying energy at lower prices and using it when the price is higher - Price Arbitrage;
- Ability to participate in DSR without having to suspend production. The Demand Side Response (DSR) service is the temporary reduction of power consumption by energy consumers at times when the continuity of energy supply is threatened by high electricity demand. Signing a commitment in which a company declares that it can temporarily reduce its power consumption at the operator's command is profitable. Distribution system operators are willing to pay as much as PLN 200,000 a year for declaring a temporary reduction in ordered power by 1 MW. Companies with a minimum of 300 kW of connection power are automatically certified for the programme. It is also possible if a minimum of 2 GWh of electricity is drawn from the grid annually. Having an energy storage facility makes it possible to earn money by participating in DSR services without restricting the operation of an industrial plant during a power reduction.
Environmental benefits:
- Reducing CO2 emissions into the environment;
- Enabling greater use of RES;
- Support for electric vehicle charging infrastructure by providing additional power during high load times.
Business benefits:
Increase the ability to win tenders by meeting criteria for the use of renewable energy sources and CO2 reduction. The EU's core criteria for green public procurement recommend including electricity supply from at least 50 per cent non-fossil sources. Where comprehensive criteria are applied, a 100% share of renewable sources is recommended. The use of weather-dependent RES alone to meet these criteria is not feasible in practice, but the cooperation of renewables with energy storage may already allow this.

Energy storage
as support for RES
The progressive energy transition means an increasing use of energy from renewable sources in the country's energy balance. However, renewable sources are weather-dependent and the characteristics of their energy generation do not coincide with the characteristics of energy demand over time. This results in destabilisation of the electricity system manifested in local voltage and frequency spikes, which in turn necessitates the shutdown of RES installations. This in turn means losses for their owners.
The solution is to use energy storage.
Energy storages enable more RES to be connected to the grid
Investors developing renewable energy sources often face refusals of permission to connect installations due to the capacity of the grid at the site of the planned investment. This is a problem in particular for those who would like to continuously develop their portfolio and achieve a greener and more economical outcome.
Energy storages allow more RES installations to be connected to the grid than the technical capacity of the connection infrastructure would allow, When energy generation exceeds demand, the excess energy is transferred to the energy store for later use, limiting the local voltage and frequency boost caused by discrepancies between generation and consumption characteristics.
Energy storages increase the utility of existing RES installations
Once the available minimum generation of coal and gas units has been exceeded and emergency exports have been agreed, system switching of generation sources occurs to balance supply and demand on the electricity system. This generates losses and extends the payback period. The use of energy storage with the possibility of island operation minimises these problems by giving greater flexibility in terms of feeding energy back into the grid.
Energy storage facilities collect some of the excess energy generated by RES and store it, allowing it to be used later at no additional cost. Stabilising the level of generation also makes it possible to adjust the timing of energy intake and return to the grid with the possibility of taking advantage of price differences due to demand. Energy storage therefore makes it possible to increase the cost-effectiveness of photovoltaic installations.
Hybrid RES installations with energy storage
A hybrid RES installation is a combination of at least two different renewable energy sources. These sources, which differ in their generation periods, offer the possibility of greater energy independence than just one of them. However, the combination of weather-dependent sources, such as solar and wind, does not guarantee constant generation. The degree of utilisation of the installed capacity of such a hybrid, imposed by law, is very high, resulting in the need for energy storage, with the share of energy injected into the electricity grid via storage in the total volume of energy injected into the electricity grid being no less than 5%. Although energy sources such as photovoltaic and wind farms often complement each other, there are times when generation can exceed the connection capacity. An essential element of this ideal symbiosis is energy storage, which makes it possible to stabilise generation and optimise a constant level of generation to the grid.

Energy storage
a power market
The capacity market is a mechanism to ensure the continuity of power supply to consumers. The Power Market Act introduces a capacity obligation, i.e. an obligation on the part of an energy producer to remain ready to supply a specific capacity to the power system. This will increase Poland's energy security. All owners of generating units with a capacity of at least 2 MW and willing owners of smaller units will take part in a certification process, after which they can participate in auctions organised by Polskie Sieci Elektroenergetyczne. This is beneficial for energy suppliers because of the remuneration they receive for their willingness to supply energy in situations where energy security becomes endangered.
Participation in the power market is also made possible by self-contained energy storage facilities which can provide adequate power when requested or when there is a need to reduce consumption from the grid - but only under favourable conditions. The stored energy in the storage allows flexible management and the fulfilment of the four-hour on-demand backup condition.

Energy storage as an indispensable part of the electricity system
In the era of the energy transition, energy storage facilities are becoming essential for the proper functioning of the electricity system. Their functions include:
- Prevention of frequency and voltage drops and spikes - frequency and voltage fluctuations adversely affect the operation of equipment and the infrastructure of the power system, leading to blackouts in parts of the system or to the disconnection of RES installations. Energy storages, as sources whose output can be controlled, can prevent this, contributing to the ecology and economy of the power system operation.
- Improving energy security - Energy security means ensuring continuity of power supply for consumers. It is threatened by failures of energy generation and transmission infrastructure. As the Energy Market Information Centre points out, energy storage facilities minimise the losses caused by failures and thus contribute to improving the country's energy security. In the future, when the Polish energy system implements more battery-based energy storage facilities, it will be possible to react instantly to failures and changes in RES generation, which will increase the operational security of the system.
- Phase voltage symmetry - asymmetry of phase voltages leads to a reduction in the efficiency of energy generation, transmission and distribution. Energy storage facilities make it possible to equalise the load on individual phases.
- Reactive power control - reactive power is consumed by many current consumers; it is essential for their proper operation. However, its consumption causes a number of adverse phenomena, such as a reduction in the generators' capacity, an increase in voltage drops and power losses in the network and a reduction in its capacity for active power. This results in the need for reactive power compensation. Energy storage units, whose inverters can operate at different power factors, will help with this.

Construction sites - back-up power supply at unconnected sites
Providing power on a construction site often involves the use of a generator. This device alone, however, is not the most ecological and economical solution, as it is associated with environmentally unfriendly exhaust emissions, noise and high fuel consumption.
The solution is to use ecological and economical power sources, which are photovoltaic panels with energy storage. The energy storage will serve to store and later use the excess energy generated by the photovoltaic installation.This minimises the use of the generator, which in such a system will only be used in the critical situation of a total lack of sunshine and the discharge of the energy storage.
The use of energy storage together with a PV installation significantly reduces carbon dioxide emissions from the fuel that powers the genset.
For environmental reasons, in many cases the noise generated by the equipment must be reduced as much as possible, which is guaranteed by the installation of the RES and the muted genset.
The operating costs of supplying the facility from the stand-alone station are minimised by the installation of the RES.
It is possible to use the equipment in multiple locations which allows the return on investment to be optimised.
Product breakdown of ZPUE energy storage facilities
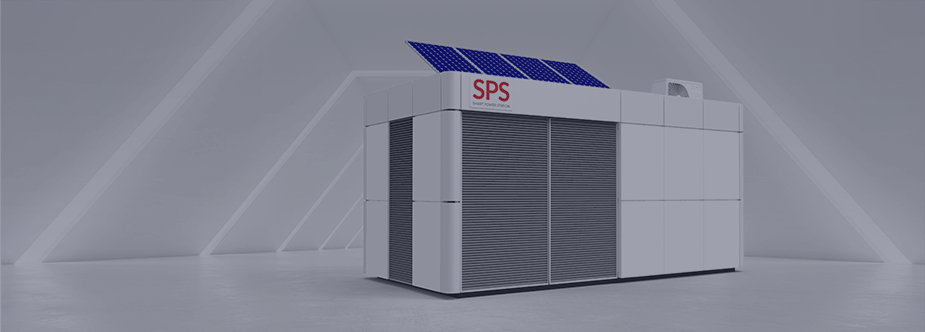
SPS - Intelligent transformer station with energy storage
The intelligent transformer station with energy storage is a solution that integrates the functions of a remotely managed distribution-distribution transformer station operating in a Smart Grid system with a bidirectional inverter cooperating with an energy storage in the form of a lithium-ion battery. The station, equipped with an internal charging station, provides the ability to charge electric vehicles. The system is complemented by the possibility of supplying energy storage or consumers directly from renewable sources of electricity, such as photovoltaic or wind farms.
Components included in a smart transformer station with energy storage:
- Energy storage,
- Two-way inverter,
- LV switchgear,
- Power transformer,
- MV switchgear (optional),
- Control cabinet SPS-Control,
- Fire detection and suppression system,
- HVAC system.
- optional components: fast chargers for electric vehicles, inverters for RES operation, extinguishing system.
Advantages of a smart transformer station with energy storage:
- The station allows for automated intelligent management of grid and RES power distribution, enabling batteries to be charged when generation exceeds needs or when grid prices are low, and discharged when prices are higher/ generation is low (price arbitrage).
- It allows power peaks to be smoothed and gives the possibility to adjust the value and direction of active and reactive power flow.
- It can be used to provide backup power in the event of a mains power failure.
- The station provides the ability to charge electric vehicles, both cars and, for example, buses, thanks to a charging station with a specific power output.
- The individual components can form independent installations. However, when managed by SPS-Control, they can also operate as a single advanced system that effectively improves the reliability of power supply to electricity facilities, optimising electricity demand and the associated financial outlay.
- The intelligent transformer station with energy storage is a fully scalable station. This means that we respond to each and every request from our customers, striving to create a facility that is fully optimised and tailored to the specific needs of the user. The common feature of the SPS substation is its two-part construction, an above-ground part and an underground part (it is possible to build the entire substation in an above-ground part).
- The possible positioning of the batteries in the underground area ensures optimum cell operating temperatures without the need for an extensive HVAC system. An additional advantage of the underground is fire safety. The housing with batteries below ground level creates natural fire barriers, which significantly improves the fire resistance of the entire station. This solution also doubles the footprint compared to a traditional above-ground solution.
- Adapting energy storage technology, e.g. lithium-ion batteries with different chemical compositions, lead-acid batteries or supercapacitors, allows the technical parameters of the energy storage to be adapted to the required application and functionality.

Energy storage in concrete enclosures

Energy storages in concrete enclosures are a versatile solution for placing an energy store next to a production facility in order to benefit from advantages such as fully exploiting the generation potential of existing RES installations, performing price arbitrage or providing an energy reserve in the event of a power failure. As the manufacturer is responsible for constructing the entire energy storage unit including the enclosure, the investor only has to provide space for its installation and enable it to be connected to existing electrical circuits.
Concrete-enclosed energy storage facilities are prefabricated containers consisting of three monolithic reinforced concrete elements made to class C30/37 - the foundation, the main body and the roof. The use of such a solution allows the walls to be constructed with adequate fire resistance. The concrete shell provides a better thermal conductivity coefficient and the additional thermal insulation significantly reduces the energy required to maintain the right temperature inside the shell.
Elements entering the energy storage in a concrete enclosure:
- Energy storage,
- Two-way inverter,
- Rozdzielnica nN,
- Power transformer,
- MV switchgear (optional),
- Control cabinet SPS-Control,
- Fire detection and suppression system,
- HVAC system.
Advantages of energy storage installed in a concrete enclosure:
- It allows the full potential of RES to be exploited.
- It can be used to provide backup power in the event of a mains power failure.
- It allows power peaks to be smoothed (limitation of ordered power) and gives the possibility to regulate the value and direction of active and reactive power flow.
- Allows price arbitrage (charging batteries when grid energy prices are low and discharging when prices are higher).
- The manufacturer is responsible for the entire construction of the energy storage including the enclosure, control system and its commissioning using the best available technology and materials of the highest quality.
- Depending on requirements, it is possible to design stations with an internal service aisle or with an external service option for energy storage with small capacities.
- It is possible to combine concrete stations with each other to achieve high-capacity energy storage in the smallest possible footprint.
- The modular design allows the energy storage to be expanded as and when required.
- More favourable temperature conditions inside the enclosure for the operation of energy storage devices.
- High weather resistance of the housing.

Energy storage in metal enclosures
Metal-enclosed energy storages are a versatile solution that allows energy storage to be placed next to the production facility and also relocated if necessary, thanks to their lightweight, easily transportable design.
The use of metal-enclosed energy storage offers benefits such as the full utilisation of the generation potential of existing RES installations, price arbitrage and the provision of energy reserves in the event of a power failure, all at any location. As the manufacturer is responsible for constructing the entire energy storage unit including the enclosure, all that is required is to provide space for its installation and to enable it to be connected to existing electrical circuits.
All cladding components: roof, walls, doors and flashings are made of polyester powder-coated aluminium sheets. Between the sheet metal panels of which the walls are made, there is additional insulation in the form of mineral wool to minimise unwanted heat exchange with the environment. The lightweight frame of the container is made of structural steel.
Elements entering the energy storage in a metal enclosure:
- Energy storage,
- Two-way inverter,
- LV switchgear,
- Power transformer,
- MV switchgear (optional),
- Control cabinet SPS-Control,
- Fire detection and suppression system,
- HVAC system.
Advantages of energy storage installed in a metal enclosure:
- It allows the full potential of RES to be exploited.
- It can be used to provide backup power in the event of a mains power failure.
- It allows power peaks to be smoothed (limitation of ordered power) and gives the possibility to regulate the value and direction of active and reactive power flow.
- Allows price arbitrage (charging batteries when grid energy prices are low and discharging at higher prices).
- Simple and rapid transport of the energy storage from site to site is possible, making it well suited as a temporary power source.
- The manufacturer is responsible for the entire construction of the energy storage including the enclosure, control system and its commissioning using the best available technology and materials of the highest quality.
- Depending on requirements, it is possible to design stations with an internal service aisle or with external service, even in the case of high-capacity energy storage, thus minimising the footprint.
- Foundation on a foundation slab ensures easy and quick installation.
- The modular design allows the energy storage to be expanded as and when required.

Energy storage in existing premises
An energy storage facility installed in an existing room is the optimum solution for customers who already have a room with dimensions that guarantee the proper positioning and ventilation of the energy storage facilities. The use of a fire detection and extinguishing system requires the energy storage room to be airtight. Installation in a room avoids the cost of housing, shortens the administrative processes for obtaining planning permission for the energy storage facility and offers the possibility of installing it close to a connection to the internal grid.
Elements included in the energy storage installed in the existing room:
- Energy storage,
- Two-way inverter,
- LV switchgear,
- Power transformer,
- MV switchgear (optional),
- Control cabinet SPS-Control,
- Optional fire detection and extinguishing system,
- Optional HVAC system.
Advantages of energy storage installed in an existing room:
- It allows the full potential of RES to be exploited.
- It can be used to provide backup power in the event of a mains power failure.
- It allows power peaks to be smoothed (limitation of ordered power) and gives the possibility to regulate the value and direction of active and reactive power flow.
- Allows price arbitrage (charging batteries when grid energy prices are low and discharging at higher prices).
- Can be installed without incurring housing costs and building conditions.
- The manufacturer is responsible for supplying the energy storage components together with the control system and its commissioning using the best available technology and materials of the highest quality.

Pole energy storage
The MEW-s Pole Energy Storage is ZPUE's answer to the connection problems of distributed generation installations. It is equipped with an intelligent algorithm that decides on the direction of power flow. The energy store is dedicated to the Distribution System Operators (DSOs) to help maintain the appropriate parameters of electricity and also enables prosumers to use the full potential of the installed Renewable Energy Source (RES). -This all without the need to secure the appropriate development conditions and go through time-consuming procedures.
Elements included in the energy storage pole:
- Energy storage,
- Two-way inverter,
- LV switchgear,
- Power transformer,
- Control cabinet SPS-Control,
- HVAC system.
Advantages of columnar energy storage:
- It allows the full potential of photovoltaic farms on the same circuit to be exploited.
- Allows full regulation of network parameters (voltage and frequency) to ensure high power quality.
- It allows power peaks to be smoothed and gives the possibility to adjust the value and direction of active and reactive power flow.
- Provides increased energy security.
- It allows price arbitrage (charging batteries when grid energy prices are low and discharging at higher prices).
- Prosumers can generate energy without disruption, giving DSOs more time to carry out energy infrastructure upgrades.
- Can be installed without building conditions, quickly and easily, without time-consuming procedures. Once the investment project (network upgrade) has been completed, the possible relocation of the storage facility to another site is relatively simple and unproblematic.
- Integrable with new or existing pole stations based on technical analysis.

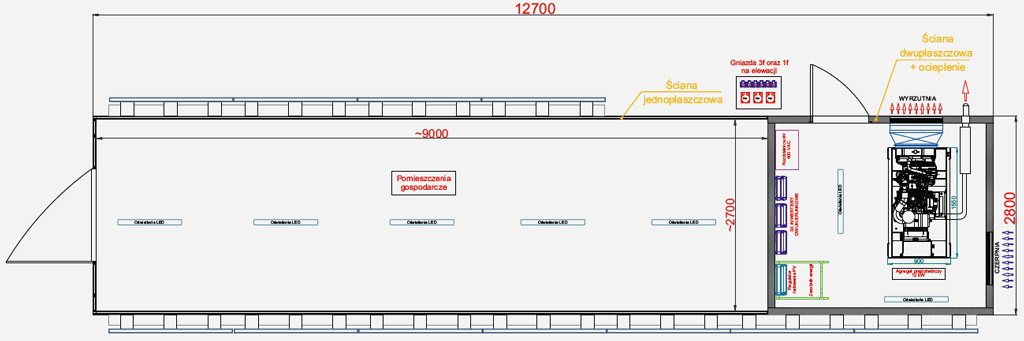
Stand-alone power supply station SPS Move
Providing power at locations without access to the mains, e.g. construction sites, often involves the use of a generator. This alone, however, is not the most environmentally friendly and economical solution, as it is associated with environmentally unfriendly exhaust emissions, noise and high fuel consumption. The solution is to use an autonomous power station, equipped with additional, ecological and economical power sources in addition to the generator, which are photovoltaic panels and energy storage. The aim of SPS Move is to minimise energy production from the fuel powering the genset and derive as much as possible from the photovoltaic installation mounted on the portable container. An essential part of such a system is the energy storage part, which allows maximum generation over time. When the system is operating on standard fuel, the energy storage allows the system to be overloaded and operate at the rated parameters of the genset.
Components of the SPS Move power station:
- Photovoltaic panels - the use of photovoltaic panels guarantees the use of environmentally friendly solar energy to power equipment. Our proposed solution will allow you to gain additional points in tenders taking into account environmental criteria. In addition, the use of free solar energy will increase the economy of the power supply. The photovoltaic panels with which the SPS Move autonomous power station is equipped are made of robust components of high quality and efficiency.
- Energy storage (battery banks and inverters) - ecological and economic impact.A photovoltaic installation alone would only allow power to be maintained in sunny weather, whereas an energy storage in the form of lithium-ion batteries made with LFP technology allows energy from photovoltaic panels to be stored when generation exceeds current demand and then used to power equipment at night or in unfavourable weather. Its operation is fully automated and adapted to optimise the use of photovoltaic energy. The energy storage can also work in conjunction with a generator.
- Generator set - when energy demand exceeds generation for an extended period of time, stored energy can run out. However, even this will not be a problem for the SPS Move power station. It is equipped with a generator which will allow power to be provided regardless of conditions. Its operation is also automated and oriented towards optimal power generation. In addition, one of its optional extras is the reduction of the sound power level at 7 m from 55 dB to 47.1 dB, so that operating in its vicinity does not cause discomfort. The genset is installed on an enclosure frame, under a roof, with external access.
- Control system - The SPS Move autonomous power station is equipped with an intelligent power distribution management system, managing all power sources (genset, PV installation and energy storage). In this way, their operation is optimised in terms of meeting the current demand and reducing as much as possible the running time of the genset in favour of utilising the capacity of the PV plant and energy storage.
- Charging station and power outlets - due to the electrification of industry, more and more equipment is powered by electricity. Electric vehicles can use the installed 22kW AC charging station, and portable electrical equipment can be powered from external three-phase and single-phase sockets located on the container façade.
Top view - equipment layout
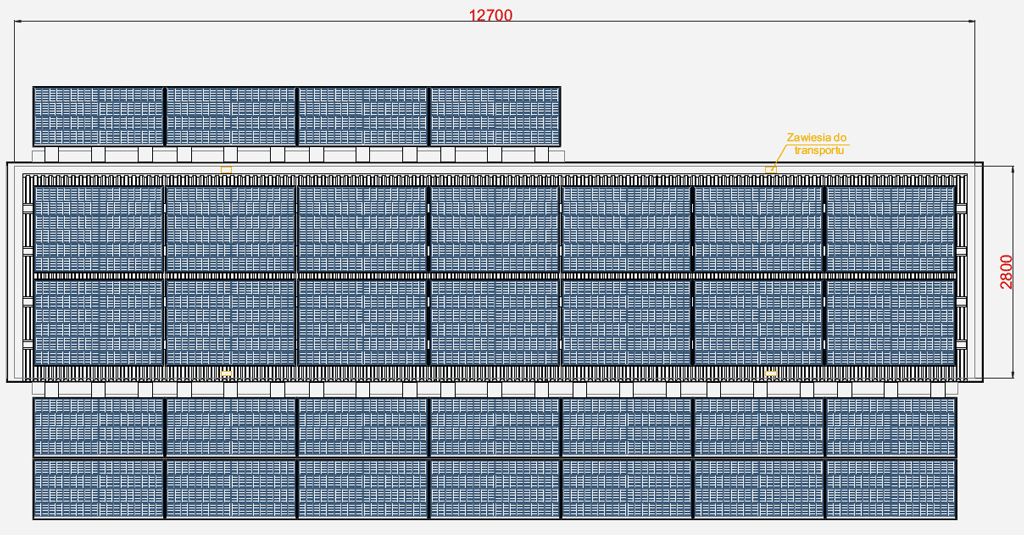
Top view - panels laid out
Advantages of SPS MOVE:
- Equipped with an intelligent power distribution management system, the SPS Move stand-alone power station provides an environmentally friendly and cost-effective power supply at off-grid locations.
- The foldable photovoltaic panels located on the roof and side walls of the SPS Move station ensure ease of transport and installation, and allow the station to take up as little space as possible.
- The energy storage unit with a bidirectional inverter and other necessary equipment is housed in an insulated room allowing optimised battery operating conditions.
- The second room can be freely used according to the client's needs, such as an office, a watchman's room or a storage room. It is possible to adapt the room by installing air conditioning and even adding doors, windows and walls. External design features such as colour and logos can also be tailored to the client's requirements.
- The station has a lightweight metal structure that not only facilitates transport, but also allows the container to be positioned at the best possible angle to the direction of the sun's rays on the photovoltaic panels (it is also possible to tilt the structure). This will maximise energy generation. It is also possible to systemically connect photovoltaic panels mounted on, for example, a neighbouring container.
- The equipment used in the SPS Move, is designed to operate as quietly as possible. If only RES installations are used, the resulting noise is generated by the inverters inside the housing. In the event of harsh weather conditions, the noise will be generated by the genset, which is designed to operate in an environment requiring noise reduction.
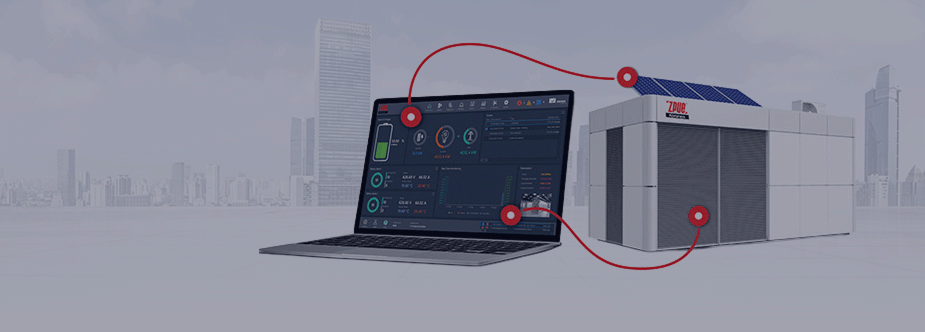
EMS energy management system - SPS Control and SPS Soft
An EMS (Energy Management System) is a programmable control system used to control and optimise the operation of energy generation and transmission systems. The EMS system is essential for exploiting the full potential of energy storage facilities. SPS Control allows the integration and communication of all the devices included in the storage and the management of their operation through suitably developed operating algorithms and functionalities. SPS SOFT is an extended energy management system that allows the integration and communication of equipment outside the storage facility and the creation of an optimised energy management facility. Communication with EMS systems is bi-directional, allowing for master operation or adaptation to an existing facility management system.
SPS SOFT system
The SPS-Soft system is designed to visualise and archive data from the Energy Storage Facility and associated equipment, and plays a key role in effectively managing and monitoring the operation of the Energy Storage Facility. Its operation can be divided into several main functions:
- Data visualisation:
- User Interface: SPS Soft offers a graphical user interface (GUI) to easily track key performance indicators of the energy storage and associated devices. This can include data such as battery charge level, power output, energy consumption, and more.
- Diagrams and Charts: The application allows data to be presented in the form of diagrams and time charts, making it easier to analyse trends and identify potential problems.
- Data archiving:
- Database: SPS Soft automatically saves the collected data to a database, which enables measurement history and retrospective data analysis. Archiving includes operational data as well as alarms or critical events.
- Reports and Analysis: The system can generate periodic reports to help assess the efficiency of the energy storage facility and plan future activities.
- Setting operating parameters:
- Configuration Parameters: Users can configure and modify the energy storage's operating parameters, allowing it to optimise its performance according to current needs or expectations. This can include charge/discharge limits, storage operation schedules and more.
- Real-time control: The system also allows real-time control of energy storage, responding to changing operating conditions or grid requirements.
- Device integration:
- Communication with Devices: SPS Soft is designed to work with a variety of energy storage-related devices, including batteries, converters, sensors and more. The system can communicate using industry standard protocols.
SPS-Control system
The SPS-Control control system is designed to supervise all the equipment installed in the energy storage facility. The system has been developed on a programmable logic controller (PLC), which makes it possible to monitor the status of the equipment and switchgear installed in the energy storage. This makes it possible to collect information and, on the basis of this information, to implement the appropriate control of equipment involved in the storage function.
Managed elements in energy storage:
- Two-way inverter,
- energy storage (e.g. battery, capacitor bank),
- MV switchgear,
- LV switchgear,
- energy meters and analysers,
- air conditioning,
- extinguishing system,
- fire detection system.
It is possible to control both each device individually and a group of devices in automatic mode, performing individual energy storage functions.
Example of the operator panel screen of the SPS-Control control system
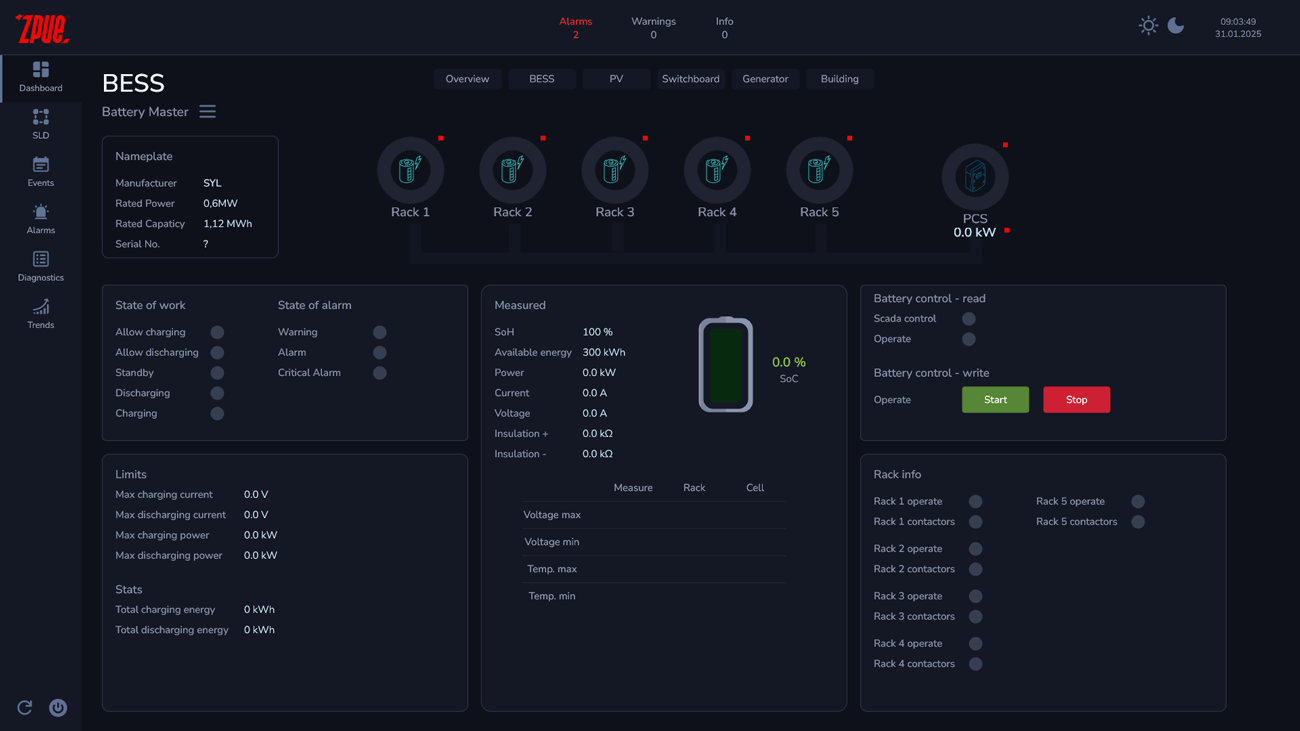
The basic functions of the SPS-Control system are:
- charging energy storage from the grid at any time (e.g. off-peak times) and discharging energy storage to loads/grid at any time (e.g. during peak times),
- charging RES energy storage during overproduction and discharging after a period of overgeneration,
- OFF grid operation,
- power reduction on demand,
- supplying energy to electric car charging stations at full capacity
Each function has appropriate parameter sets prepared to allow the operator to change the settings.
The intelligent algorithms of the SPS-Control control system are specially developed functions that, through appropriate parameterisation, allow optimum control of the actuators to improve the performance of the network or to gain economic benefits through energy storage and return. Among the most popular are:
- smoothing of the load curve,
- stabilisation of network parameters on the low voltage side,
- reactive power compensation,
- distortion compensation (harmonics),
- Voltage regulation with active and reactive power,
- stabilisation of the power of anxious receivers,
- Stabilisation of RES power.


















